So this is my 100th post, and I felt like it should be kind of special. So I want to cover a question I get a lot, and one that’s important to me; what exactly is materials science? My early answer was that “it’s like if physics and chemistry had a really practical baby.” One of my favorite versions is a quote from this article on John Goodenough, one of the key figures in making rechargeable lithium ion batteries: “In hosting such researchers, Goodenough was part of the peculiar world of materials scientists, who at their best combine the intuition of physics with the meticulousness of chemistry and pragmatism of engineering”. Which is a much more elegant (and somewhat ego-boositng) way of wording my description. In one of my first classes in graduate school, my professor described materials science as “the study of defects and how to control them to obtain desirable properties”.
A more complete definition is some version of the one that shows up in most introductory lessons: materials science studies the relationship between the structure of a material, its properties, its performance, and the way it was treated. This is often represented as the “materials science tetrahedron”, shown below. Which turns out to be something people really love to use. (You also sometimes see characterization float in the middle, because it applies to all these aspects.)

The materials science tetrahedron (with characterization floating in the middle).
Those terms may sound meaningless to you, so let’s break them down. In materials science, structure goes beyond that of chemistry: it’s not just what makeup of an atom or molecule that affects a material, but how the atoms/molecules are arranged together in a material has a huge effect on how it behaves. You’re probably familiar with one common example: carbon and its various allotropes. The hardness of diamond is partially attributed to its special crystal structure. Graphite is soft because it is easy to slide the different layers across each other. Another factor is the crystallinity of a material. Not all materials you see are monolithic pieces. Many are made of smaller crystals we call “grains”. The size and arrangement of these grains can be very important. For instance, the silicon in electronics is made in such a way to guarantee it will always be one single crystal because boundaries between grains would ruin its electronic properties. Turbine blades in jet engines and for wind turbines are single crystals, while steels used in structures are polycrystalline.
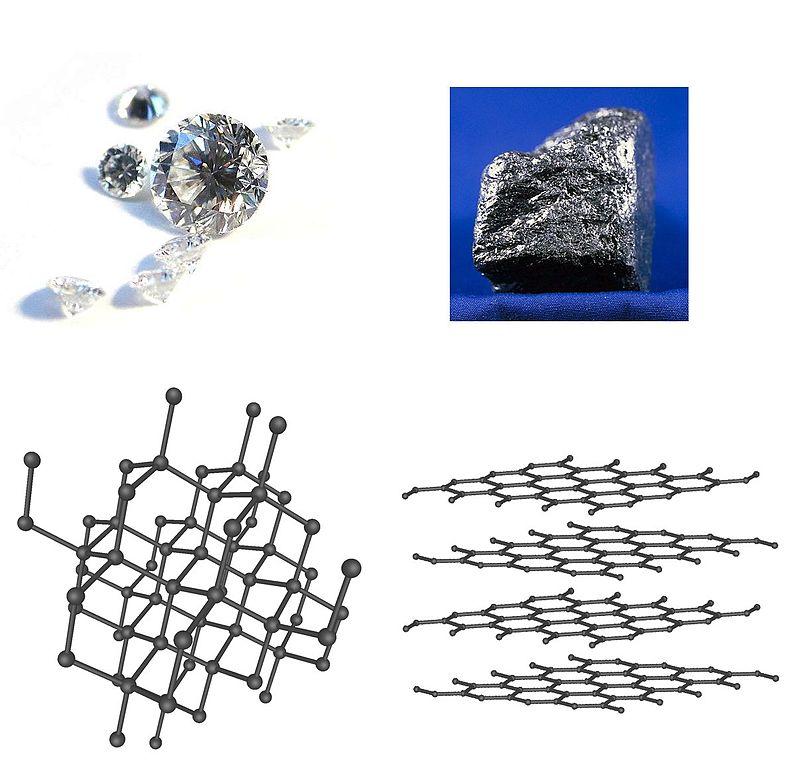
Diamond and its crystal structure is on the left; graphite on the right.
Processing is what we do to a material before it ends up being used. This is more than just isolating the compounds you’ll use to make it. In fact, for some materials, processing actually involves adding impurities. Pure silicon wouldn’t be very effective in computers. Instead, silicon is “doped” with phosphorus or boron atoms and the different doping makes it possible to build various electronic components on the same piece. Processing can also determine the structure – temperature and composition can be manipulated to help control the size of grains in a material.
Properties and performance are closely related, and the distinction can be subtle (and honestly, it isn’t something we distinguish that much). One idea is that properties describe the essential behavior of a material, while performance reflects how that translates into its use, or the “properties under constraints“. This splits “materials science and engineering” into materials science for focusing on properties and materials engineering for focusing on performance. But that distinction can get blurred pretty quickly, especially if you look at different subfields. Someone who studies mechanical properties might say that corrosion is a performance issue since it limits how long a material could be used at its desired strength. Talk to my colleagues next door in the Center for Electrochemical Science and Engineering and they would almost all certainly consider corrosion to be a property of materials. Regardless, both of these depend on structure and processing. Blades in wind turbines and jet engines are single crystals because this reduces fatigue over time. Structural steels are polycrystals because this makes them stronger.
Now that I’ve thought about it more, I realize the different parts of the tetrahedron explain the different ways we define materials science and engineering. My “materials science as applied physics and chemistry” view reflects the scale of structures we talk about, from atoms that are typically chemistry’s domain to the crystal arrangement to the larger crystal as a whole, where I can talk about mechanics of atoms and grains. The description of Goodenough separates materials science from physics and chemistry through the performance-driven lens of pragmatism. My professor’s focus on defects comes from the processing part of the tetrahedron.
The tetrahedron also helps define the relationship of materials science and engineering to other fields. First, it helps limit what we call a “material”. Our notions of structure and processing are very different from the chemical engineers, and work best on solids. It also helps define limits to the field. Our structures aren’t primarily governed by quantum effects and we generally want defects, so we’re not redundant to solid-state physics. And when we talk about mechanics, we care a lot about the microstructure of the material, and rarely venture into the large continuum of mechanical and civil engineers.
At the same time, the tetrahedron also explains how interdisciplinary materials science is and can be. That makes sense because the tetrahedron developed to help unify materials science. A hundred years ago, “materials science” wouldn’t have meant anything to anyone. People studying metallurgy and ceramics were in their own mostly separate disciplines. The term semiconductor was only coined in a PhD dissertation in 1910, and polymers were still believed to be aggregates of molecules attracted to each other instead of the long chains we know them to be today. The development of crystallography and thermodynamics helped us tie all these together by helping us define structures, where they come from, and how we change them. (Polymers are still a bit weird in many materials science departments, but that’s a post for another day)
Each vertex is also a key branching off point to work with other disciplines. Our idea of structure isn’t redundant to chemistry and physics, but they build off each other. Atomic orbitals help explain why atoms end up in certain crystal structures. Defects end up being important in catalysts. Or we can look at structures that already exist in nature as an inspiration for own designs. One of my professors explained how he once did a project studying turtle shells from an atomic to macroscopic level, justifying it as a way to design stronger materials. Material properties put us in touch with anyone who wants to use our materials to go into their end products, from people designing jet engines to surgeons who want prosthetic implants, and have us talk to physicists and chemists to see how different properties emerge from structures.
This is what attracted me to materials science for graduate school. We can frame our thinking on each vertex , but it’s also expected that we shift. We can think about structures on a multitude of scales. Now I joke that being a bad physics major translates into being great at most of the physics I need to use now. The paradigm helps us approach all materials, not just the ones we personally study. Thinking with different applications in mind forces me to learn new things all the time. (When biomedical engineers sometimes try to claim they’re the “first” interdisciplinary field of engineering to come on the scene, I laugh thinking that they forget materials science has been around for decades. Heck, now I have 20 articles I want to read about the structure of pearl to help with my new research project.) It’s an incredibly exciting field to be in.